
TECHNICAL DOCUMENTATION
REGULATORY DOCUMENTATION
 Along with our own regulatory requirement for registration of products, our regulatory department also offer services for Product Registration and Dossiers in CTD format. Our Pharmaceutical Regulatory Services includes services for GMP, Drug Master File (DMF), Validation, Training and Documentation, Mock audits and other regulatory requirements including generation of technical data. International Regulatory requirements & generation of technical data in line with the regulatory requirements and submission of dossiers for various country specific requirements managed by international level team.
Along with our own regulatory requirement for registration of products, our regulatory department also offer services for Product Registration and Dossiers in CTD format. Our Pharmaceutical Regulatory Services includes services for GMP, Drug Master File (DMF), Validation, Training and Documentation, Mock audits and other regulatory requirements including generation of technical data. International Regulatory requirements & generation of technical data in line with the regulatory requirements and submission of dossiers for various country specific requirements managed by international level team.
TECH TRANSFER
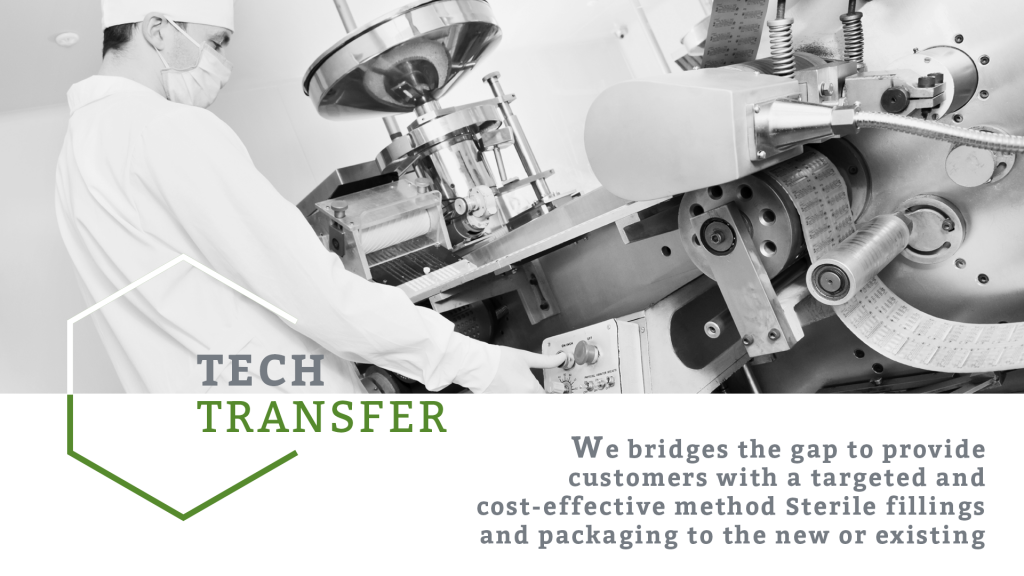 Our goal is to help other sterile formulations manufacturing companies to achieve cost effective sterile formulations manufacturing technology starting from sterile fillings to final secondary packaging. At Gulf Biotech, our experienced staff in engineering, quality control and research & development work on tech transfer project on Sterile Liquid Injectable products development and manufacturing. Our experts will take the responsibility for entire transfer process from early stage development to process optimization to late stage validation and commercial execution. Gulf Biotech can meet all necessary process needs in order to achieve the most efficient and highest quality manufacturing, as well as ensure its success. We cover a verity of product types including lyophilized Vials, Cartridges and ampoules.
Our goal is to help other sterile formulations manufacturing companies to achieve cost effective sterile formulations manufacturing technology starting from sterile fillings to final secondary packaging. At Gulf Biotech, our experienced staff in engineering, quality control and research & development work on tech transfer project on Sterile Liquid Injectable products development and manufacturing. Our experts will take the responsibility for entire transfer process from early stage development to process optimization to late stage validation and commercial execution. Gulf Biotech can meet all necessary process needs in order to achieve the most efficient and highest quality manufacturing, as well as ensure its success. We cover a verity of product types including lyophilized Vials, Cartridges and ampoules.
We bridges the gap to provide customers with a targeted and cost-effective method Sterile fillings and packaging to the new or existing companies aiming to extent their product portfolios. We also assists or clients in developing and improving their existing liquid injectable products and manufacturing processes to enhance their market competitiveness and to improve the quality of products.